Drug for treatment of vascular anomalies
January 27, 2021
Background / Context / Abstract:
Vascular anomalies are broadly classified into vascular tumors and vascular malformations. Most of them are of unknown cause and radical treatment methods have not been established. The present inventors discovered earlier that a gel preparation containing sirolimus (rapamycin) can be used to treat skin lesions associated with tuberous sclerosis complex and successfully developed it as a medicinal drug (sirolimus gel). After that, the inventors found that sirolimus and sirolimus derivatives are effective for the treatment of vascular anomalies as a result of careful examination of the gel. The therapeutic drug for vascular anomalies in the present invention has been completed.
Technology Overview:
During the trials of the sirolimus gel, the present inventors found that sirolimus was effective in constricting dilated blood vessels. Moreover, the addition of sirolimus to melanocytes significantly decreased the expression of HIF1 alpha (hypoxia-inducible factor) and sirolimus suppressed the production of VEGFs (vascular endothelial growth factors) in various cells in a concentration-dependent manner. Therefore, sirolimus was shown to have not only a tumor-suppressive effect but also an antiangiogenic effect. It was also found that early topical application of sirolimus gels to patients with vascular malformation can prevent exacerbation of vascular malformation with less adverse reactions.
Benefits:
The gel in the present invention is easier to administer compared to the conventional oral agent. It can be administered at the patient’s home, etc. and requires no frequent hospital visits. In addition, the gel can be administered directly to a lesion and the concentration of the active ingredient in the lesion can be maintained at a high level. When the gel is applied to a lesion, the active ingredient is present at a high concentration in the lesion, but the concentration of the active ingredient in blood is maintained at a relatively low level. Thus, this enables safe treatment with less adverse effects on the body and less adverse reactions.
Potential Applications / Potential Markets:
We will grant a license from this university to a company and aim at practical application after the conduct of clinical trials by the company.
State of Development / Opportunity / Seeking:
・Available for exclusive and non-exclusive licensing
・Exclusive/non-exclusive evaluation for defined period (set up for options)
・Collaborative/supportive research
※Seeking
1. Development partner
2. Licensing
IP Status:
WO2020/175131
Figures:
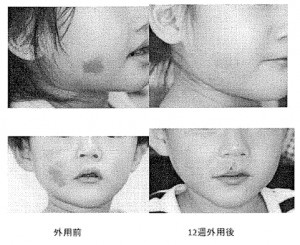
Results of the administration of sirolimus gel in two patients with erythema due to vasodilatation and increased capillary blood vessels under the epidermis
Contact:
![]()